Outline
We are engaged in the research about electromagnetic energy.
We try to reveal the physical and chemical behavior of the electromagnetic energy with noble functional nano-hybrid systems and apply this unique effect to
chemical reaction system, material creation, material synthesis. Specifically, we are handlimg
such as microwave region of the electromagnetic wave (1mm-1m), and near ultraviolet, visible and near
infrared region of the electromagnetic wave (100nm-1000nm), which are written in following section.
Innovation of Chemical Reaction Process with Microwave Chemistry
Acceleration of Chemical Reaction by Microwave Heating
When the microwave is penetrating into the material, electromagnetic energy is converted into heat energy, as a familiar example, the microwave oven. The microwave has unique characteristics, internal heating and material selection heating. We use these Microwave effects to acclerate the solid catalyst reaction.Results 1 We synthesized a core-shell type zeolite and this core was filled with the carbon. We used this core-shell zeolite to the catalyst of alcohol dehydration reaction under microwave irradiation. We were able to show that carbon was heated efficiently and dehydration raction was promotied by Microwave with high temperature field. (J. Catal., in press)
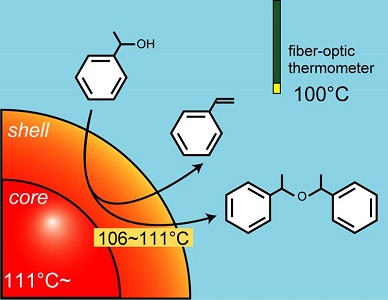
Results 2 When the dehydrogenation of ethylbenzene using a magnetite as a catalyst was carried out under microwave irradiation, the temperature of the central portion of the solid catalyst had the highest status. In this endothermic dehydrogenation reaction, it can be seen that it has become a favorable inversion temperature distribution, and this unique phenomenon was studied in detail by simulation analysis.
Acceleration of Electron Transfer by Microwave Special Effect
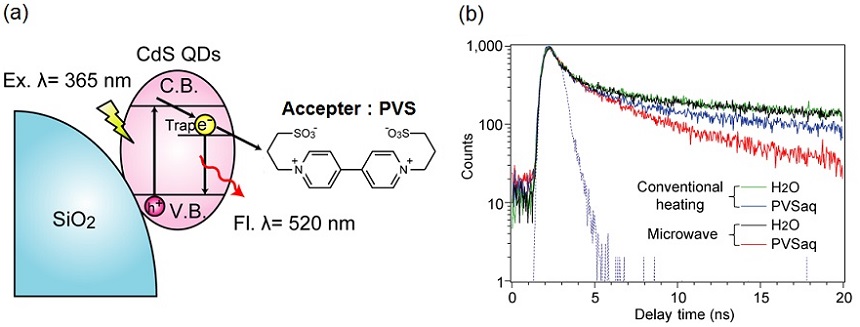
Oxidation-reduction reaction is industrially very important. Some of the aldehyde synthesis reactions by the alcohol oxidation with a solid oxidizing agent(e.g. the manganese oxide and the chromium oxide...), examples of such oxidation-reduction reaction, are known to be accelerated under microwave irradiation. We revealed that the electron transfer reaction process which is an important elementary reaction process of the oxidation-reduction reaction are accelerated under microwave irradiation.Result 3 Photoinduced electron transfer rate from the cadmium sulfide to the electron acceptor molecule is determined by photoluminescence measurement of cadmium sulfide, which proved that the electron transfer rate was accelerated under microwave irradiation (Fig. 2).
Result 4 We found an acceleration of electron transfer reduction reaction from Ni metal particles to the electron acceptor molecule under microwave irradiation. In this system, we got the analysis result that this acceleration of electron transfer was attributed to the oscillation of the alternating electric field or the alternating magnetic field of the microwave.
Control of Photo-Induced Electron Transfer with Nano-Designed Structure
Photo-Induced Charge Separation in the Alternately Layered Structure
The nano-hybrid system which is composed of arranged nano-particles or nano-sheet as higher nano-ordered structures enables to control the electron transfer which is the most important elementary reaction process in the photocatalyst, the dye-sensitized solar cell or the perovskite solar cell. We have been promoting the following studies on the new materials, higher-ordered array structures.Result 5 In the alternated layered structure with the titanium oxide nanosheets and the tungsten oxide nanosheets stacking via alkyl chains, one way electron transfer occurred in according to each of the electronic band structure under light irradiation. And we showed that this electron transfer rate depended on interlayer length, which was equivarent to electron transfer distance (Fig. 3).
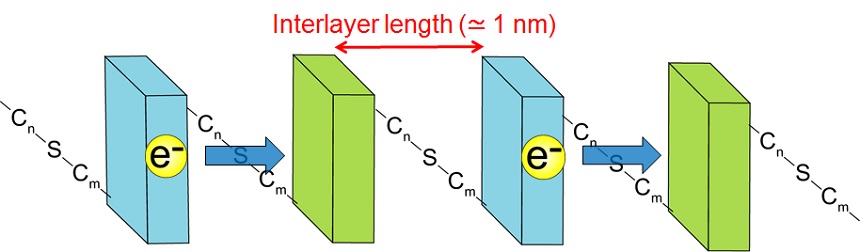
Result 6 We expanded the Result 5 to various types of metal oxide semiconductors, and we showed that the electron transfer rate also depended on the band structure of the metal oxide semiconductors. In the other words, the electron transfer rate also depanded on energy gap of the band.
Nano-Designed Structures in Dye-Sensitized or Perovskite-Sensitized Solar Cells
Result 7 We found that electron injection process from dye to titanium oxide nanoparticle which concluded (001) plane was accelerated varied with higher ratio of the (001) plane (Fig. 4).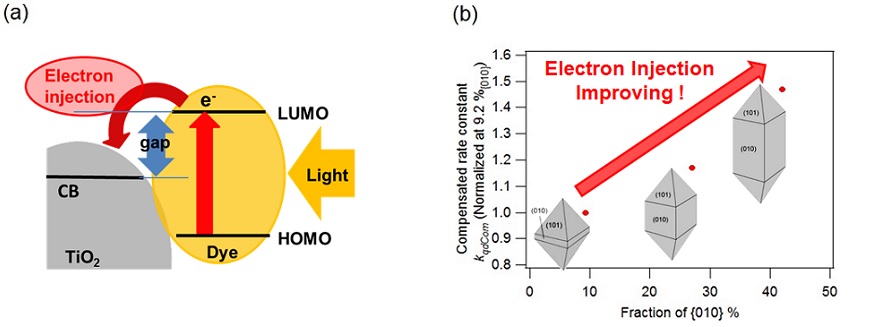
Result 8 We developed a technique for arranging the titanium oxide which concluding high ratio of (001) plane to the surface on the substrate. And We applyed this array structure to dye-sensitized or perovskite-sensitized solar cells.







